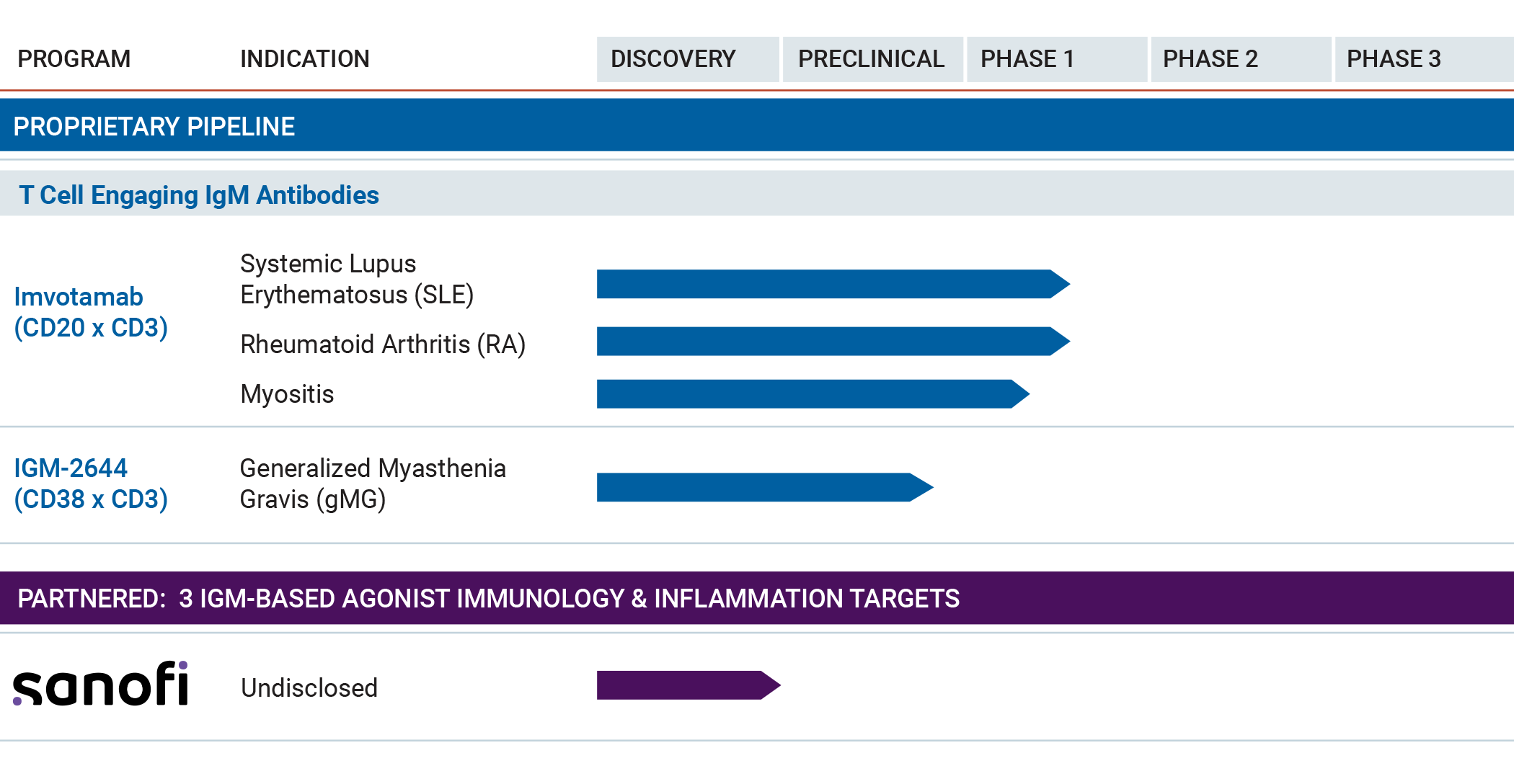
We have a robust pipeline that demonstrates the breadth and depth of our science.

IgM Antibodies and Autoimmune and Inflammatory Diseases
Antibody treatments have revolutionized outcomes for patients with autoimmune and inflammatory diseases, including rheumatoid arthritis, psoriasis, and others.
Autoantibody-driven diseases represent a subset of the broader autoimmune category of diseases, and include systemic lupus erythematosus, rheumatoid arthritis, myasthenia gravis, and pemphigus vulgaris. Chronic treatment is required for symptom management and disease control. Many of these diseases are progressive and are associated with significant morbidity and impact on quality of life.
Imvotamab is a bispecific T cell engaging IgM antibody targeting CD20 and CD3 proteins and is in Phase 1b clinical trials in severe systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and idiopathic inflammatory myopathies (myositis).

Research & Discovery at IGM
Our ability to develop engineered IgM antibodies against various targets allows for a broad and differentiated product pipeline. We have robust research and pre-clinical development plans for our IgM antibodies in autoimmune and inflammatory diseases.
Clinical Trials
With our new class of medicines, we are conducting clinical trials for our initial product candidates to evaluate the potential benefit of these novel treatments in patients.
Expanded Access
IGM Biosciences recognizes that some patients with serious or immediately life-threatening diseases may not be eligible for participation in a clinical trial and may have exhausted all available treatment options. Subject to internal review and approval based on the conditions described in IGM Biosciences’ Individual Patient Expanded Access (IPEA) Policy, IGM Biosciences may grant individual patients access to an unapproved or investigational product outside of a clinical trial setting through our IPEA program. IPEA requests are sponsored by a healthcare professional and applies only to therapies under an active Investigational New Drug (IND) application.
For more information, please read our Individual Patient Expanded Access Policy.