
IgM Antibodies and Autoimmune and Inflammatory Diseases
Antibody treatments have revolutionized outcomes for patients with autoimmune and inflammatory diseases, including rheumatoid arthritis, psoriasis, and others.
Autoantibody-driven diseases represent a subset of the broader autoimmune category of diseases, and include systemic lupus erythematosus, rheumatoid arthritis, myasthenia gravis, and pemphigus vulgaris. Chronic treatment is required for symptom management and disease control. Many of these diseases are progressive and are associated with significant morbidity and impact on quality of life.

Research & Discovery at IGM
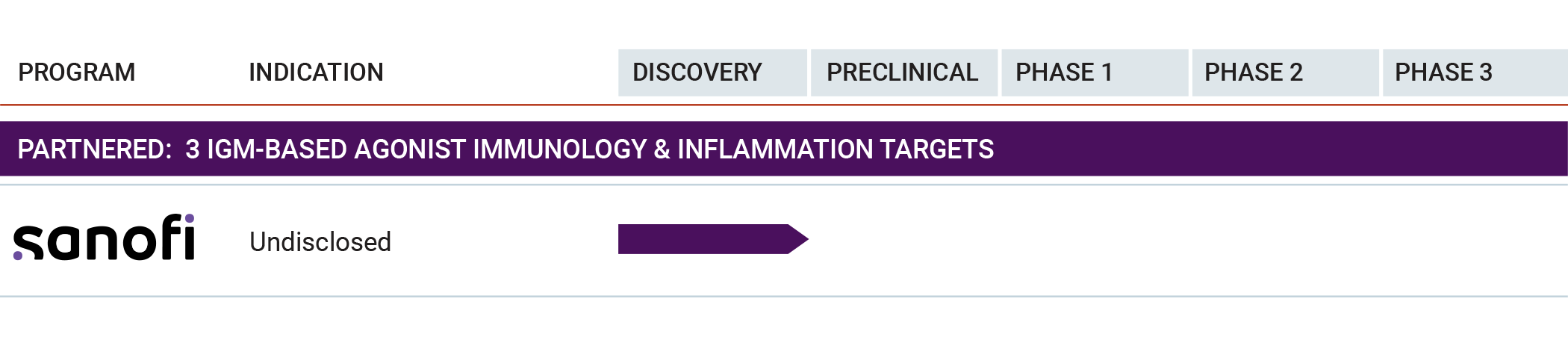
Our ability to develop engineered IgM antibodies against various targets allows for a broad and differentiated product pipeline. We have robust research and pre-clinical development plans for our IgM antibodies in autoimmune and inflammatory diseases.